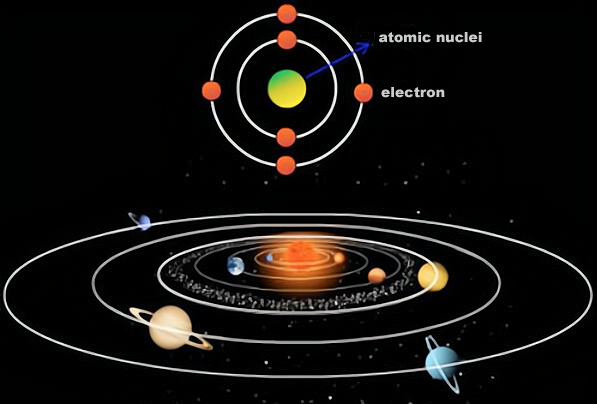
An atom is made up of a nucleus and electrons, while a nucleus is made up of neutrons and protons. For an atom, the number of protons in its nucleus determines the type of element it is. For example, the element hydrogen with an atomic number of 1 has only one proton in its nucleus, helium with an atomic number of 2 has two protons in its nucleus, and so on for other elements.

So in theory, if we can keep adding protons to a nucleus, it will become a heavier and heavier element. Of course, after all, protons are positively charged and they don't like each other, which creates a strong repulsive force, so we need to add a certain number of neutrons along with the protons to keep the nucleus stable.
This is easier said than done, so much so that humans with modern technology cannot do it. But just because humans cannot do it does not mean that the universe cannot do it, otherwise there would not be a wide variety of elements in the universe, so how does the universe do it? One common mechanism is nuclear fusion.

In simple terms, nuclear fusion is the aggregation of lighter atomic nuclei into heavier nuclei at high temperatures and pressures. Every star in the universe is a natural "Fusion reactor", and under the pressure of its own gravity, the core of the star will form a high temperature and pressure environment, thus providing the conditions for nuclear fusion.
The higher the atomic number of the nucleus, the higher the conditions for fusion, and the temperature and pressure in the core of a star are proportional to the mass of the star, so those stars in the universe with low masses will not be able to fuse.
For example, the star next door to our solar system, proxima, is actually a very low-mass red dwarf, which can only fuse hydrogen into helium, and a yellow dwarf like the sun, which can only fuse up to oxygen with an atomic number of 8 over the lifetime of the sun.
Only stars of sufficient mass have a core capable of initiating round after round of fusion reactions to produce heavier and heavier elements, but even such stars cannot fuse all the elements known in the universe, because the fusion of stars ends at iron with an atomic number of 26.
In other words, at the core of the star, iron is the end of nuclear fusion. Why is this so? Because the fusion of iron nuclei does not release energy, but absorbs it.
The energy released by nuclear fusion is the basis for a star to maintain its stability, and for the huge stars in the universe that fuse iron, their own gravity is so strong that if the fusion at their core stopped releasing energy, the star would be "Crushed" By its own gravity and this is also known as a supernova explosion.
So the question arises, since fusion ends at iron, where do the heavier elements in the universe come from? The answer is "Neutron capture".
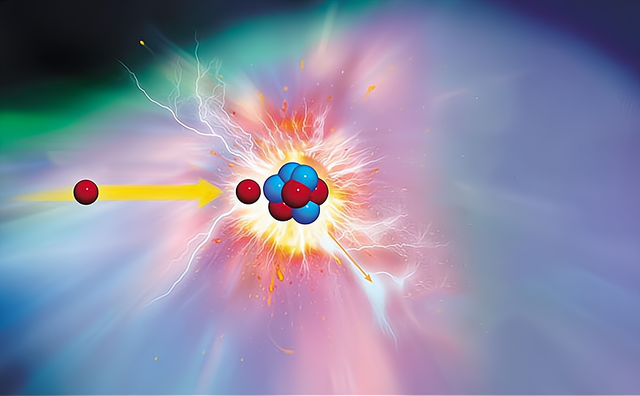
"Neutron capture" Is the capture of neutrons by the nucleus of an atom. To make it easier to understand, we can imagine the nucleus of an atom as an "Eater", and in an environment with neutron radiation, these eaters may "Eat some of them can "Eat" Several neutrons, while others can "Eat" Only one neutron and "Eat" The other. Some of them can "Eat" Several neutrons without any problem, while others can "Eat" Only one neutron and get "Indigestion".
For example, if an fe-56 nucleus "Eats" A neutron, it becomes fe-57, and since fe-57 is a stable isotope, it is fine. In the following time, if it "Eats" Another neutron, it becomes fe-58, which is still a stable isotope, so it still doesn't matter.
If it "Eats" Another neutron, it becomes the unstable fe-59, so it has "Indigestion", in which case the nucleus of fe-59 undergoes beta decay, in which a neutron in its nucleus decays into a proton, and the nucleus decays into a proton. In this process, a neutron in the nucleus decays to a proton and releases an electron and an antineutrino, adding 1 to its atomic number, which then becomes a cobalt-59 nucleus.
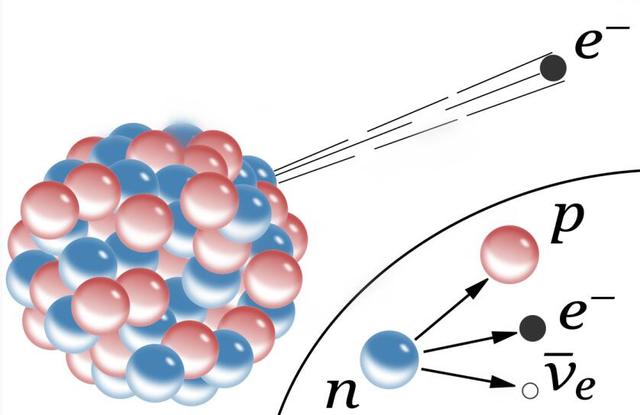
Compared to the iron-56 nucleus, the cobalt-59 nucleus is much less "Digestible" And after "Eating" A neutron, it becomes the unstable cobalt-60, so it also undergoes beta decay and its atomic number is again increased by 1, and then the nickel-60 nucleus is more "Digestible" And "Eats" Three neutrons in a row before it undergoes beta decay and becomes a copper-63 nucleus... ...
This "Neutron capture", as described above, usually occurs in the interiors of stars, which are also known as "Slow neutron capture" Because of the relatively weak neutron radiation in the interior of the star and the relatively low efficiency with which heavy elements are produced.
There is "Slow neutron capture" In the universe, and of course there is also "Fast neutron capture", but in fact, of all the elements known to be heavier than iron in the universe, the contribution of "Slow neutron capture" Is actually in fact, of all the elements known in the universe that are heavier than iron, the contribution of "Slow neutron capture" Is not really significant, and it is "Fast neutron capture" That really produces them in large quantities.

When high-energy events such as supernova explosions and neutron star collisions occur in the universe, they create an environment of extremely high neutron radiation in a short period of time, which can be as high as 100 trillion neutrons per cubic centimetre per second.
In such a high neutron density environment, "Fast neutron capture" Occurs, where the lighter nuclei are "Gobbled up" And then suffer severe "Indigestion", so that they decay in various ways, and when things settle down, there are a lot of elements heavier than iron in the universe.