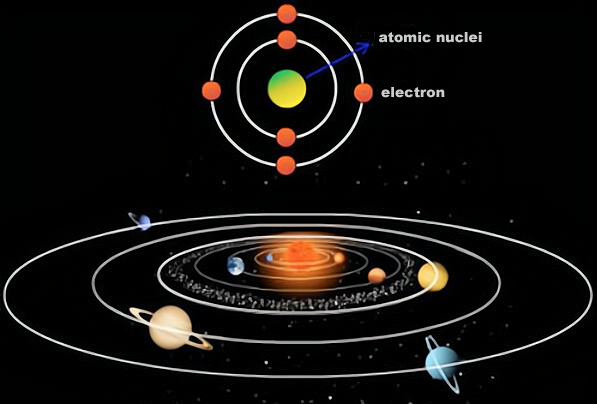
In the macroscopic world, the solar system is a system of celestial bodies centred on the sun, in which the vast majority of mass is concentrated and the major planets orbit the sun, while in the microscopic world, an atom is a microscopic system centred on the nucleus of an atom, in which the vast majority of mass is concentrated and electrons orbit the nucleus.
This similarity between the solar system and the atom is reminiscent of the fact that, even in the observable universe, the solar system is negligibly small, so if we look at it on a more macroscopic level, could the solar system be an atom? In which the sun is the nucleus and the planets are the electrons?
On the face of it, there seems to be some truth to this hypothesis - after all, the structure of the solar system is so similar to that of an atom - but in physical terms, there are actually huge differences between the two.
1、Fundamental forces and electric charge
There are four fundamental forces in the universe, namely gravity, electromagnetism and strong and weak interactions, of which strong and weak interactions exist only at the level of the atomic nucleus.
In the solar system, the dominant fundamental force is gravity, with the major planets of the solar system orbiting the sun under the influence of gravity, while inside the atoms, the dominant force is electromagnetism, where the effect of gravity is largely negligible (because the masses of the nuclei and electrons are so small).
Furthermore, if the solar system were really an atom, then the sun, the "Nucleus", would only have a positive charge, and the planets, the "Electrons", would only have a negative charge, which is clearly not the case in the solar system.
2、 Mass
In the standard model of particle physics, electrons are elementary particles, and they all have the same mass (as early as the 20th century, physicists have accurately measured the mass of an electron to be 9.10956 x 10^-31 kg).
This means that if the solar system is an atom, then the masses of the major planets in the solar system should also be the same. However, we all know that there are considerable differences in the masses of the major planets in our solar system, for example, the most massive planet (jupiter) is about one thousandth the mass of the sun, the least massive planet (mercury) is about 6 millionth the mass of the sun, and the earth is again about 330,000th the mass of the sun.
3、 Energy

The sun is constantly releasing light and heat, and its energy comes from fusion reactions in its core, whereas this is not the case with atomic nuclei, which in fact can only release energy in the form of decay without interference from external particles, and this release of energy is not sustainable.
Incidentally, the nucleus may also release electrons or anti-electrons in the process of decay, i.e. If the sun were a nucleus and decayed, it could release planets or "Anti-planets" In the process... ...
4、 Motion
At first, it was thought that the electrons within an atom moved in the same way as the planets around the sun, this was called the 'planetary model', but this was disproved as research progressed.
In fact, electrons have "Wave-particle duality" And their motion is random and uncertain (a common phenomenon in the microscopic world), so we cannot predict exactly where an electron will appear at the next point in time, but only how likely it is to appear at a particular location.
Scientists therefore often describe the movement of electrons within an atom in terms of the probability of their appearance per unit volume, which appears as if the nucleus is enveloped in a cloud-like structure, which has come to be known as the "Electron cloud".
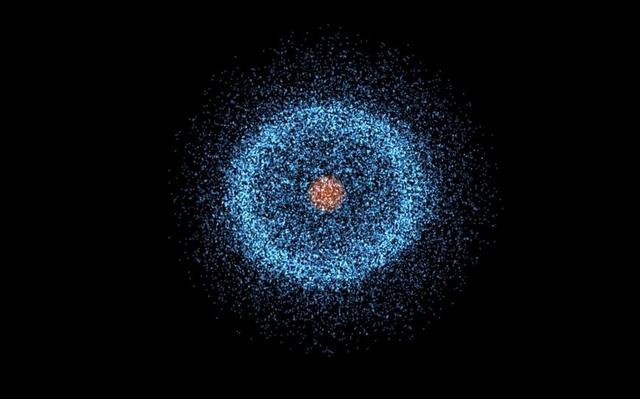
In the solar system, the motion of the planets is deterministic and they move around the sun in an orderly manner, without enveloping it in an 'electron cloud'.
In fact, we can always predict where the planets will be at the next point in time based on their current state of motion, and with enough observations, we can even predict exactly when and where special phenomena such as solar and lunar eclipses will occur.
It is clear from the above that there are huge differences, both between the sun and the nucleus, and between planets and electrons. It can therefore be said that, even on a more macroscopic level, the solar system would not be an atom.